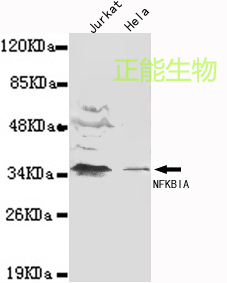
-
IKB-α 编辑
中文名:人核因子κB抑制蛋白α
外文名:IKB-α
Preferred Names:
NF-kappa-B inhibitor alpha
NF-kappa-B inhibitor alpha
ikB-alpha
IkappaBalpha
I-kappa-B-alpha
nuclear faCTor of kappa light chain gene enhancer in B-CELLs
major histocompatibility complex enhancer-binding protein MAD3
NF-kappa-B inhibitor alpha NF-kappa-B inhibitor alpha ikB-alpha IkappaBalpha I-kappa-B-alpha nuclear factor of kappa light chain gene enhancer in B-cells major histocompatibility complex enhancer-binding protein MAD3
IKB-α的 基因定位于14号染色体长臂1区3亚区的2号位上
Summary:This gene encodes a member of the NF-kappa-B inhibitor family, which contain multiple ankrin repeat domains. The encoded protein interacts with REL dimers to inhibit NF-kappa-B/REL complexes which are involved in inflammatory responses. The encoded protein moves between the cytoplasm and the nucleus via a nuclear localization signal and CRM1-mediated nuclear export. Mutations in this gene have been found in ECTodermal dysplasia anhidrotic with T-cell immuNOdeficiency autosomal dominant disease.
Protein function:Inhibits the activity of dimeric NF-kappa-B/REL complexes by trapping REL dimers in the cytoplasm through masking of their nuclear localization signals. On cellular stimulation by immune and proinflammatory responses, becomes phosphorylated promoting ubiquitination and degraDAtion, enabling the dimeric RELA to translocate to the nucleus and activate transcription.
蛋白序列:
translation="MFQAAERPQEWAMEGPRDGLKKERLLDDRHDSGLDSMKDEEYEQ
MVKELQEIRLEPQEVPRGSEPWKQQLTEDGDSFLHLAIIHEEKALTMEVIRQVKGDLA
FLNFQNNLQQTPLHLAVITNQPEIAEALLGAGCDPELRDFRGNTPLHLACEQGCLASV
GVLTQSCTTPHLHSILKATNYNGHTCLHLASIHGYLGIVELLVSLGADVNAQEPCNGR
TALHLAVDLQNPDLVSLLLKCGADVNRVTYQGYSPYQLTWGRPSTRIQQQLGQLTLEN
LQMLPESEDEESYDTESEFTEFTEDELPYDDCVFGGQRLTL"
核因子-KB(nuclear factor-kappa B,NF-KB)蛋白家族是一种多效性的转录因子,可以与多种基启动子部位的KB位点发生特异性的结合从而促进其转录表达。其受氧化应激、细菌脂多糖,细胞因子等多种刺激而活化后,能调控前炎症性细胞因子、细胞表面受体、转录因子、粘附分子等的生成。而这些刺激因素及其调控的因子与微循环障碍的发生、发展均有着密切的关系。
IKB蛋白家族成员有IKBα(MAD-3,pp40)、IKBβ、IKBγ/p105、IKBδ/p100、IKBε、Bcl-3以及果蝇属的Cactus等。其家族结构特点是均有多个约33个氨基酸的重复序列,称为崐SWI6/锚蛋白重复序列,主要参与与Rel蛋白的RHD相互作用。
IKB蛋白主要有以下三个部分构成:
1.与蛋白降解有关的N-末端区;
2.能与NF-KB相互作用的内部区(区内含有锚蛋白重复序列ANK)(家族共有结构)主要参与与Rel蛋白的RHD相互作用;
3.称为PEST的C-端区,主要参与“囚禁”NF-KB在细胞浆中。
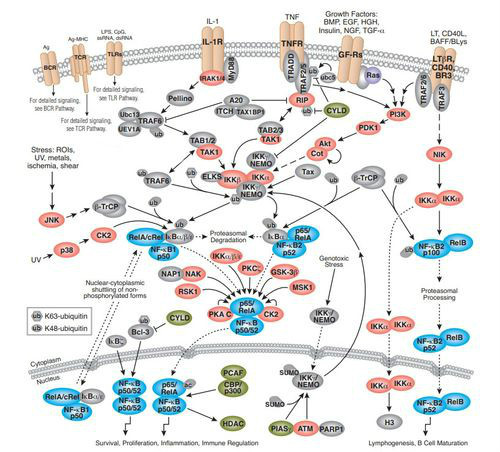
IKBs家族参与两个信号通路
NF-kB signal pathway
NF—KB存在于多种细胞内, 是一种具有多向性调节作用的核转录因子,参与炎 症、免疫、细胞增殖和细胞凋亡等多种生理、病理过 程的基因调控。
NF-KB系统由NF—KB家族及其抑制 物IKB家族共同组成。前者由Rel蛋白家族成员构 成,以同源或异源二聚体的形式存在。哺乳动物细 胞中有5种NF—KB/Rel家族成员:RelA(p65)、RelB、 C-Rel、P50(NF-KB1)和p52(NF-KB2),其N端均含 有一段保守的约300个氨基酸残基的Rel同源结构 域,该结构域包含Rel蛋白间聚合位点及与IKB结合 的位点,同时也包含DNA识别序列和核定位序列。 只有NF-KB蛋白二聚体才具有DNA结合活性,不同 NF-KB/Rel蛋白二聚体具有不同的结合序列,识别不 同的DNA靶目标,从而调节不同基因的表达。
NF-KB的激活必须通过两个步骤:①IKB从NF-KB复 合体中解离并降解,暴露NF-KB的核定位序列; ②NF-KB发生核易位,并与特异的KB序列结合。
经典途径:某些配体(TNF、IL-1、Lsp、CD 40L等)与细胞表面相应受 体结合通过胞质侧结构域募集到一些接头蛋白可以 激活经典途径,这些接头蛋白能够募集并激活IKB 激酶复合物(inhibitor of kappa B kinase complex,IKK),激活 后的IKK复合体将IKB的32和36位两个保守的丝 氨酸残基磷酸化,磷酸化的IKB发生多泛素化,最终 由26S蛋白体降解,被释放的NF-KB发生核易位,作用于相应的靶基因。
非经典途径:与经典途径不同的是:信号相 关接头蛋白只能被一些有限的信号分子,如淋巴细 胞毒素B与CD40L激活,且并不需要泛素蛋白的参 与。信号分子与受体的结合将激活NF-KB诱导激 酶,活化的激酶磷酸化IKK-α,继而将p100磷酸化, 磷酸化的p1O0部分水解后转变为p52,其核定位信 号和DNA结合结构域暴露,可与Rel-B组成复合体 进人胞核激活靶位点。
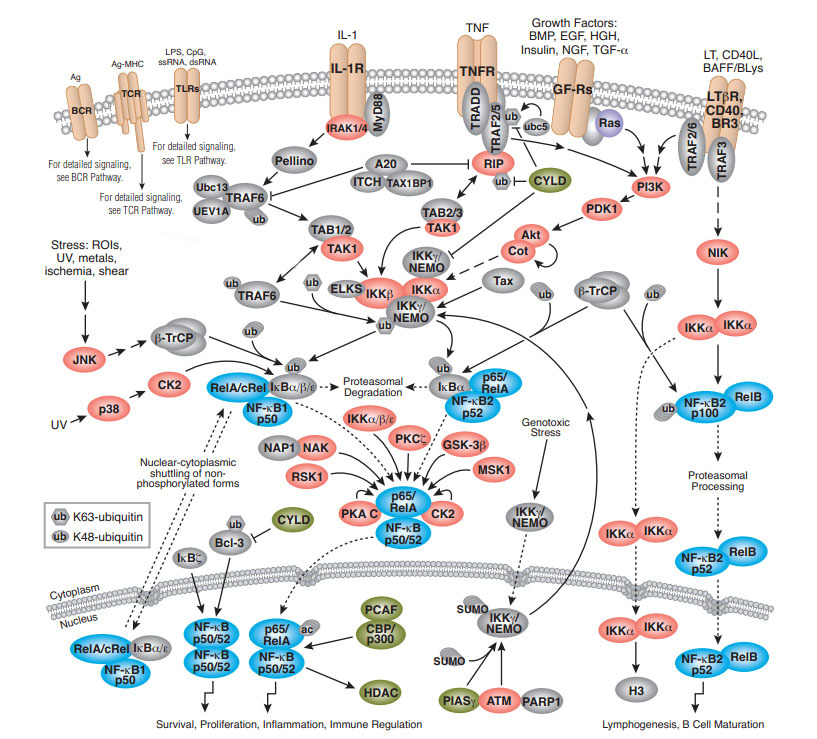 IKB-α
IKB-α
Toll-like Receptor signal pathway
类Toll受体(Toll-like receptors, TLR) 是最古老最保守的免疫系统的组成部分。所以Toll-like receptor信号通路主要作用是调控一些参与免疫的蛋白表达。
TLR多表达于哺乳动物的抗原提呈细胞以及上皮细胞, 被认为是具有相同结构的I型跨膜型模式识别受体(pattern recognition receptors, PRR) 。
PRR由紧密相连的三个部分组成:
1、胞外段是富含亮氨酸重复序列(leucine2richrepeat , LRR)的N端,约550~980个氨基酸组成;
2、跨膜区是富含半胱氨酸的结构域;
3、 胞内段与IL21受体同源, 被称为TIR(Toll/ IL21R) , 约200个氨基酸大小。LRR结构域是所有PRR共有的结构特点, 它识别广泛存在于病原体细胞表面的分子标志, 如酵母细胞壁表面上的甘露糖, 以及脂多糖、多肽糖、胞壁酸等各种细菌的细胞壁成分等,这些分子被统称为病原相关分子模式(pathogen-associated molecular patterns,PAMP) 。两者结合启动信号转导过程。TIR结构域在细胞内介导信号传递, 在蛋白之间相互作用过程中意义重大, 与天然免疫和获得性免疫作用的发挥紧密相连
lTLRs家族有13个蛋白,TLR1-9为人鼠共有,TLR10人独有,TLR11-13鼠独有。其中TLR1,TLR2,TLR4,TLR5,TLR6位于细胞表面,TLR3,TLR7,TLR8,TLR9是定位于核内体/溶酶体。
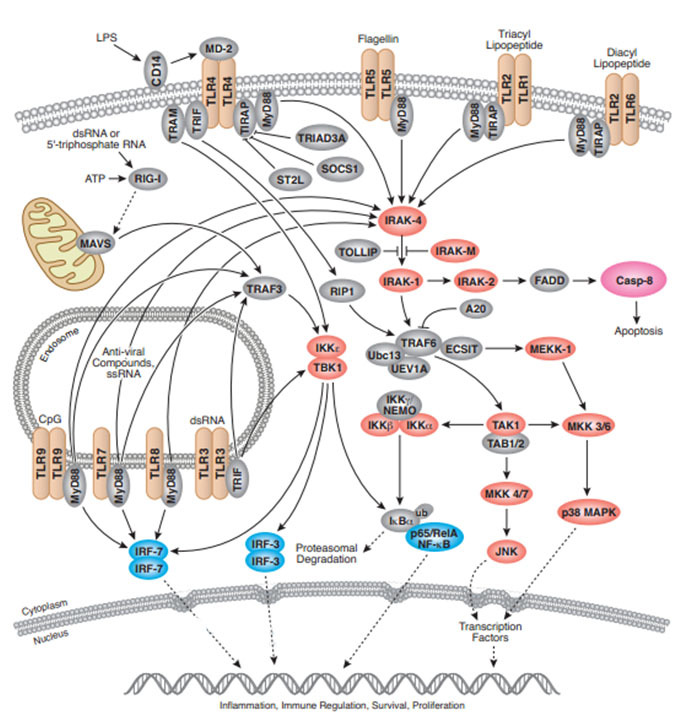 IKB-α
IKB-α
Novel 2-Phenyl-1-Pyridin-2yl-Ethanone (PpY) Based Iron Chelators Increase Expression of IkBα and Heme Oxygenase-1 and Inhibit HIV-1
HIV-1 transcription is activated by HIV-1 Tat protein, which recruits CDK9/cyclin T1 and other host transcriptional co-activators to the HIV-1 promoter. Tat itself is phosphorylated by cell cycle kinase 2 (CDK2) and inhibition of CDK2 by small interfering RNA or iron chelators inhibits HIV-1 transcription. HIV-1 transcription is also activated by NF-kB that binds to HIV-1 LTR independent to Tat but can also be recruited Tat-dependently by CDK9/cyclin T1. Recently, induction of heme oxygenase-1 (HO-1) by hemin was shown to inhibit HIV-1 in vitro and in vivo. Here, we analyzed the effect of novel phenyl-1-pyridin-2yl-ethanone (PPY) based iron chelators, PPYeT and PPYaT, on HIV-1. Both chelators efficiently inhibited one round of HIV-1 replication in T cells at low nanomolar IC50s without exhibiting cytotoxicity at 24 hrs incubation. The iron chelators efficiently bound intracellular labile iron as it was determined in calcein binding assays. Because we previously showed that iron chelators inhibited the activity of CDK9, we analyzed expression of several cellular genes dependent on CDK9. Unexpectedly, chelators were found to induce the expression of IkBα, an inhibitor of NF-kB (Fig1). Treatment with the iron chelators retained NF-kB in cytoplasm of the treated cells suggesting reduction in NF-kB in nucleus (Fig2). The chelators were also shown to induce HO-1 expression in cultured monocytes, likely to do a decrease of intracellular iron pool. This effect of iron chelators mimicked the effect of hemin treatment which also induced HO-1 and inhibited HIV-1 infection in our experimental conditions. Low nanomolar IC50s for the PPY-based iron chelators and lack of toxicity suggest their potential usefulness as future anti-retroviral therapeutics. Further studies are needed to investigate additional targets for iron chelators in HIV-1 life cycle that may include reverse transcription and capsid assembly. Therefore iron chelators need to be carefully assessed not only to understand the mechanism but also as a therapeutic strategy.